Determination of the Formula Unit of a Compound
Determine its formula unit. 2Approximately 02g of zinc is placed into the crucible and the crucible with its embrace is weighed.
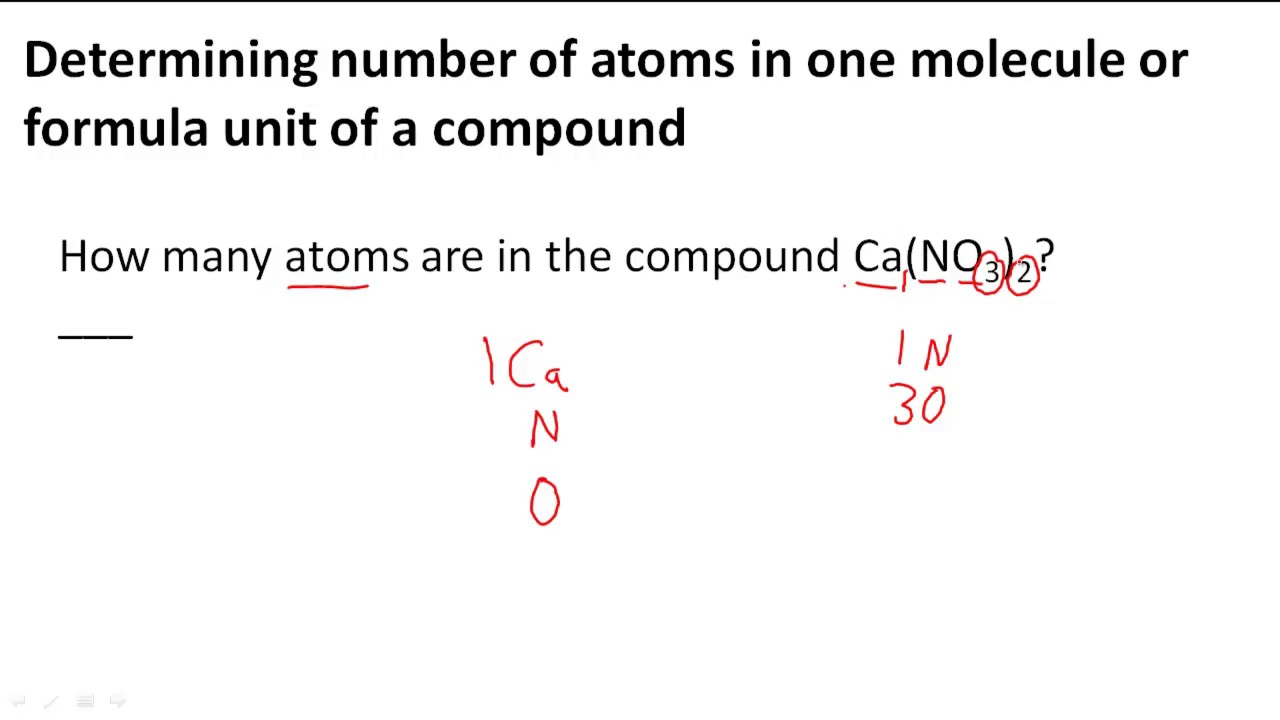
Determining Number Of Atoms In One Molecule Or Formula Unit Of A Compound Youtube
NURUL SABRINA BINTI YUSTRI.
. But the calculated formula unit is 2 Zn and 3 Cl so the formula unit will be Zn2Cl3. Formula unit of a compound is the simplest compound that can be found in a compound. A compound of 01 mole of silver and 01 mole of bromine for example will have the formula unit AgBr.
There is a difference because the experiment was done in an open place where there is air presence and we weigh balance also not in a close condition so there is. Determination of the formula unit of a compound. Chemistry Experiment 1 of the 1st semester of Matriculation.
2Approximately 02g of zinc is placed into the crucible and the crucible with its contain is weighed. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The lawful lump of zinc is determined310 ml of 6M HCl answer is poured into a measuring cylinder.
The theorecal formula unit will be 1 Zn and 2 Cl so the theoretical formula unit is ZnCl2. List the safety cautions in this experiment. The formula unit of a compound can be determined if the composition or the ratio of the elements in the compound is known.
DETERMINATION OF THE FORMULA UNIT OF A COMPOUNDpdf - NAME. Here the formula unit is -CH2--This is the simplest compound that can be found in polyethylene. Weight of the crucible zinc chloride at 1st heating 5238 g Weight of the crucible zinc chloride at 2nd heating 5237 Weight of the crucible zinc chloride at 3rd heating 5235 Emperical Formula Element Zn Mass g 025 No of Mole 025 65 000385 00056 Ratio 1 Emperical Formula 2.
The formula unit can be determined once the compounds composition is understood. The formula unit of a compound can be determined if the composition or the ratio of the elements in the compound is known. The crucible is weighed and the exact mass is recorded.
For example consider polyethylene. But the calculated formula unit is 2 Zn and 3 Cl so the formula unit will be Zn2C l 3. The exact mass of zinc is determined310 ml of 6M HCl solution is poured into a measuring cylinder.
There is a difference because the experiment was done in an open place where. To synthesis a zinc chloride compound. Consider a compound that contains 020 mole of aluminum and 030 mole of oxygen.
To determine the formula unit of zinc chloride HYPOTHESIS The empirical formula will be ZnCl 2 APPARATUS Analytical balance Crucible Tripod stand Bunsen burner Glass rod Wire gauze Measuring cylinder 10mL Porcelain Tiles MATERIALS Zinc powder HCl 60 M PROCEDURE 1. Element Al O Mole mol 020 030 Mole Ratio 1 15 Simplest Ratio 2 3 Formula unit Al 2 O 3 Updated. Determination of the formula unit of a compound.
Determination of the formula unit of a compound Essay. Determine its formula unit. Generating Alternatives For Determination Of The Formula Unit Of A Compound Case Solution.
By measuring the volume and calculating the mass of CO2. The crucible is weighed and the lawful lump is recorded. Approximately 02g of zinc is placed into the crucible and the crucible with its contain is weighed.
Up to 3 cash back The theorecal formula unit will be 1 Zn and 2 Cl so the theoretical formula unit is ZnCl2. A firm like Determination Of The Formula Unit Of A Compound must organize its management systems processes policies and strategies to fully utilize the resources potential to be valuable rare and costly to imitate. A simple chemical compound of zinc and chlorine will be created in this experiment.
1The crucible is weighed and the exact mass is recorded. Approximately 02g of zinc is placed into the crucible and the crucible with its hold is weighed. In addition we need to find out the mass of CO2 to be able to calculate its molar mass using the second formula Mmn M is a molar mass with the measuring unit grams per mole gmol m is a mass of a compound in grams g n is a number of molesmol HYPOTHESIS 1.
A quantitative analysis can be done to figure out what a compounds composition is. View LAB REPORT CHEMISTRY EXP 1DETERMINATION OF THE FORMULA UNIT OF A COMPOUNDpdf from CHE 015 at Perak Matriculation College. Consider a compound that contains 020 mole ofaluminum and 030 mole of oxygen.
NURUL SABRINA BINTI YUSTRI CLASS. Its structure is --CH2CH2CH2--base n. The exact mass of zinc is determined.
Determination of the formula unit of a compound sample essay 1The crucible is weighed and the direct concretion is commemorative.
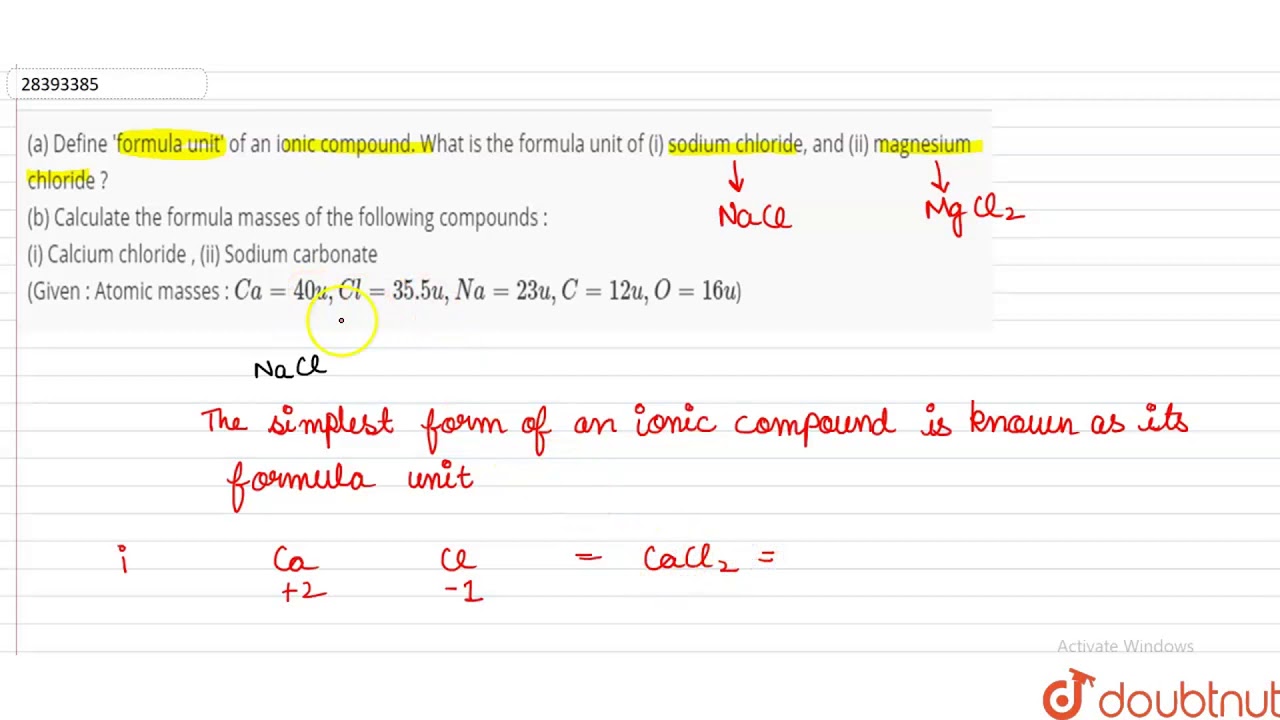
A Define Formula Unit Of An Ionic Compound What Is The Formula Unit Of I Sodium Chloride A Youtube

Empirical Formula And Molecular Formula Introduction Youtube

Write Equation From Two Points Worksheet With Model Problems Explained Step By Step Writing Equations Algebra Equations Math Formula Chart

Writing Chemical Formulas For Ionic Compounds Youtube
0 Response to "Determination of the Formula Unit of a Compound"
Post a Comment